Which of the Following Acids Has the Weakest Conjugate Base
Identify the strongest acid. Up to 256 cash back The strongest acid will generate the most stable conjugate base while the weakest acid will lead to formation of the least stable conjugate base.

Solved Which Of The Following Acids Has The Weakest Chegg Com
So OH is the conjugate base of H₂O.

. HIO3 Ka 017 I believe the answer is HCN it has the lowest Ka and is the weakest acid. Or you can say that the strongest acid yields the weakest conjugate base and the weakest acid yields the strongest conjugate base Use this strategy to explain the following pKa values. Therefore HBr is the strongest acid and its conjugate base is weakest rather than HF.
HCl is the strongest acidic and Cl is weakest conjugate base. Chemistry questions and answers. 2-hydroxybenzoic acid pKa 30.
Which of the following weak acids has the strongest conjugate base. You can argue in a. HCl is a strong acid.
Acetic acid CH3COOH is a weak acid. Acid-3 K a 3 x 10 -6. Conjugate bases are those species left when a molecule loses a hydrogen ion.
In this example of HFH2O H-COOH and HCN the pKa Values are 314 140 375 921 respectively. 1 Acid CH3CH2CH2NH3 Conjugate base CH3CH2CH2NH2- 2 Acid CH3CH2CH2OH2 Conjugate base CH3CH2CH2OH- Are my answers correct. Which of the following acids has the weakest conjugate base.
Which of the following acids will have the strongest conjugate base. Acidity increases across a period and down the group. Chemistry Please check List the conjugate base for the following acids.
So HF is the strongest acid among the given acids. Strong acids have weak bonds to their H. Complete the following table by calculating the missing entries.
So H₂CO₃ is the conjugate acid of HCO₃. D hydrocyanic acid Ka 40X10-10. A HOCI b CH3COOH c HNO2 d HF e HNO3.
A moderately weak Brønsted-Lowry acid has only a slight tendency to donate a proton. If HCl is a strong acid it must be a good proton donor. Solution for Which of the following acids will have the weakest conjugate base.
The right answer is HBr. It is called a base since it can theoretically gain a hydrogen ion back since bases are hydrogen ion acceptors. From either the Lewis or Bronsted-Lowry definition we thus come to the same conclusions.
Strong bases have a weak conjugate acid. Here HCl is a strong acid thus Cl attracts the H atom less efficiently so that it can be easily dissociated. Base acid Conj A Conj B.
Which of the statements below is FALSE. Moderately Weak Acid - Moderately Weak Conjugate Base Pair. Malonic acid pka 285 benzoic acid Ka 631 x 10-5 O Cascorbic acid pka 41 LED pyruvic acid Ka 28 x 10-3 O E.
Weak bases have a strong tendency to get H andor donate an electron pair. This makes its conjugate base Cl aq a very weak base. The stronger the acid the weaker its conjugate base.
H C l H X 2 O H X 3 O X C l X. Though Fluorine is more electronegative the bond bewteen H-F is strongest due to size match between F and H and it cant easily ionizable. Some of the acetic acid ethanoic acid molecules dissociate.
Start your trial now. Moderately weak acids include CH 3 COOH HNO 2 H 2 S NH 4 HCO 3-. Benzoic acid Ka 631 x 10-5 b.
3-chlorobenzoic acid pKa 38 d. In water it loses its acidic hydrogen to the water molecule as H ion. So the weakest conjugate base will be the F- ion HF---------H F-.
Strong bases have a weak tendency to get H andor donate an electron pair. Which of the following acids has the weakest conjugate base. Label the acid base conjugate acid and conjugate base in the following reaction.
HCO₃ H₂O H₂CO₃ OH. The Relative Strengths of Conjugate Acid-base Pairs Strong acids have a weak conjugate base. Thus the Cl - ion must be a weak base.
It has one more H atom and one more charge -1 1 0. The conjugate base of an acid is the anion that results when the acid molecule loses its hydrogen to a base. Correct option is D Strongest acid Weakest conjugate base.
Which acid has the weakest conjugate base. HOCl Ka 30 x 10-8 c. Ascorbic acid pKa 41 c.
Its tendency to reaccept its lost proton is weak. Weve got the study and writing resources you need for your assignments. Which of the following acids has the weakest conjugate base in aqueous solution.
The conjugate base of a weak acid is. CH3COOH H2O CH3COO - H3O The acetate ion CH3COO- thus formed is the conjugate base of acetic acid. We see that HCO₃ becomes H₂CO₃.
Acid-4 K a 1 x 10 -4. The reaction results in a change in oxidation number on the B and N atoms and can be described as a redox reaction. HCl can only be a good proton donor however if the Cl - ion is a poor proton acceptor.
Which acid has the weakest conjugate base. Conversely the weakest acid will give the strongest conjugate base. First week only 499.
When Cl is a conjugate base in the products side the Cl has the same low efficiency of attracting H atom. HF HBr HClO CH3COOH HNO2. The H₂O becomes OH.
Identify the weakest acid. Chloroacetic acid Ka 126 x 10-3 e. Solve any question of Equilibrium with-.
It has one less H atom and one more charge. Which of the following acids has the weakest conjugate base in aqueous solution. 3-chlorobenzoic acid pKa - 38.
4 Pts Acid-1 K a 2 x 10 -5. Acid-2 K a 4 x 10 -7. Consequently it accepts the H X ions less efficiently.
This reaction represents a Lewis acid-base reaction in which BF 3 is the Lewis acid and NH 3 is the Lewis base. For example acetic acid ethanoic acid is a moderately weak acid in aqueous solution. AHbr BHF CHNO2 DCH3COOH EHOCl.
Chemistry questions and answers. H2O CN OH HCN. A Which one of the following acids has the weakest conjugate base.
Weak acids have strong bonds to their H.
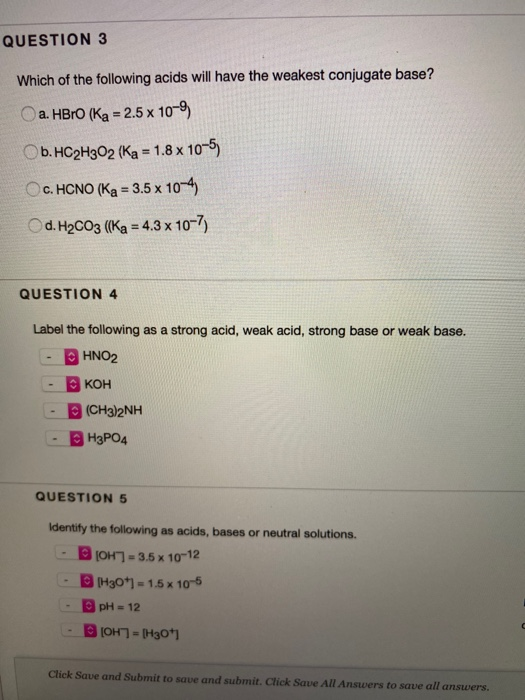
Solved Question 3 Which Of The Following Acids Will Have The Chegg Com

Solved Which Of The Following Acids Has The Weakest Chegg Com

Which Of The Following Bronsted Acids Has The Wekest Conjugate Base
Comments
Post a Comment